Emergency Use Authorization
The EUA is supported by the Secretary of Health and Human Service’s declaration that circumstances exist to justify the emergency use of medical devices during the COVID-19 pandemic.
It needs to be understood that the use of designated devices available under the EUA program has not undergone the same type of review as an FDA-approved or cleared device.
Click to see: About Emergency Use Authorizations
The FDA may issue an EUA when certain criteria are met, which includes that there are no adequate, approved or available alternatives. In addition, the FDA decision is based on the totality of scientific evidence available showing that it is reasonable to believe that the device may be effective for use by healthcare practitioners.
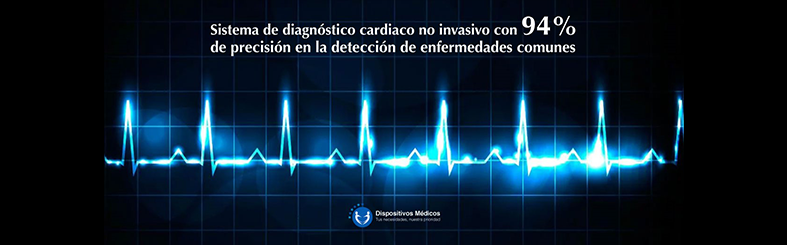
For CAPS this would mean its use as a diagnostic aid to screen for potential cardiac conditions associated COVID-19 or underlying cardiac conditions that may affect clinical management of COVID-19 adult patients with confirmed or suspected COVID-19 in patient care situations, including telemedicine and primary care setting and that the known and potential benefits of CAPS, for such use, outweigh the known and potential risks.
Click to see: Expansion of Telemedicine Services
A EUA is only in effect for the duration of the COVID-19 declaration justifying emergency use of the product unless terminated or revoked (after which the product may no longer be used).